March 2025
North America News
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from 01 February 2025 to 28 February 2025 are summarized below:
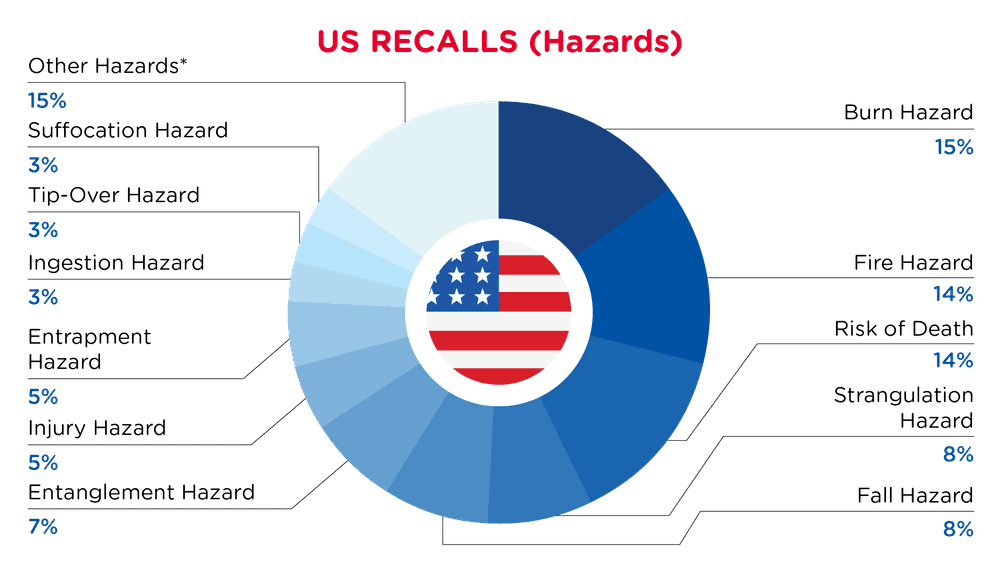
| Hazards | Frequency |
| Burn Hazard | 9 |
| Fire Hazard | 8 |
| Risk of Death | 8 |
| Strangulation Hazard | 5 |
| Fall Hazard | 5 |
| Entanglement Hazard | 4 |
| Injury Hazard | 3 |
| Entrapment Hazard | 3 |
| Ingestion Hazard | 2 |
| Tip-Over Hazard | 2 |
| Suffocation Hazard | 2 |
| Other Hazards* | 9 |
*Other Hazards include Carbon Monoxide Poisoning Hazard, Crash Hazard, Crushing Hazard, Laceration Hazard, Lead Poisoning Hazard, Amputation Hazard, Impact Hazard, Electric Shock Hazard, Poisoning Hazard and Lead Poisoning Hazard with a frequency of less than 2.

| Product Categories | Frequency |
| Furniture | 8 |
| Sporting Goods / Equipment | 7 |
| Fabric / Textile / Garment / Home Textile | 6 |
| Home Electrical Appliances | 5 |
| Electrical Appliances | 4 |
| Computer / Audio / Video / Other Electronics & Accessories | 3 |
| Toys and Childcare Products | 3 |
| Tools and Hardware | 1 |
| Outdoor Living Items | 1 |
For a complete list click here
In Canada, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Health Canada website, which is updated daily. The Canada recalls from 01 February 2025 to 28 February 2025 are summarized below:
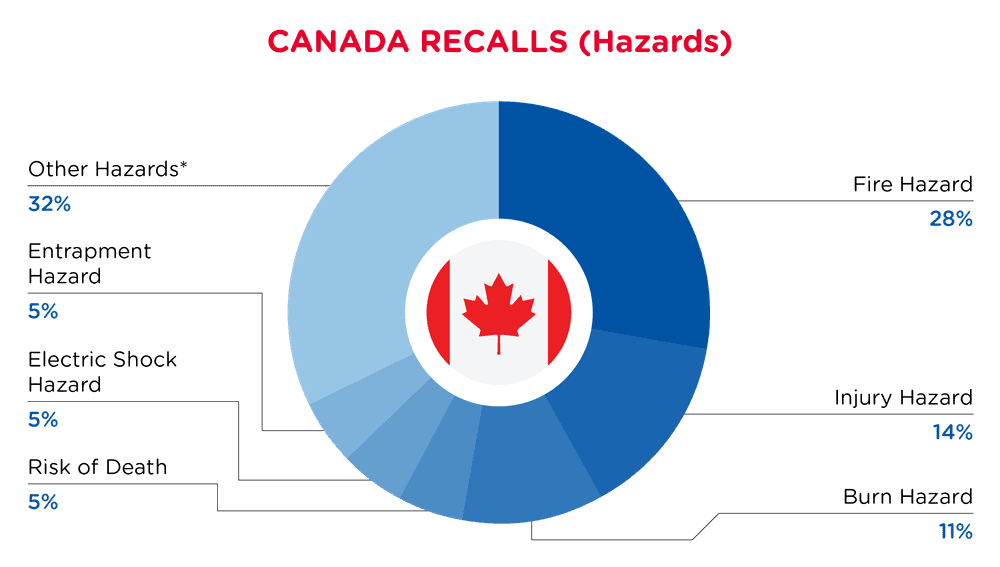
| Hazards | Frequency |
| Fire Hazard | 10 |
| Injury Hazard | 5 |
| Burn Hazard | 4 |
| Risk of Death | 2 |
| Electric Shock Hazard | 2 |
| Entrapment Hazard | 2 |
| Other Hazards* | 12 |
*Other Hazards include Skin Irritation Risk, Microbiological Hazard, Crash Hazard, Ingestion Hazard, Eye Irritation Risk, Tip-Over Hazard, Laceration Hazard, Health Risk Hazard, Aspiration Hazard, Impact Hazard, Fall Hazard and Poisoning Hazard with a frequency of less than 2.

| Product Categories | Frequency |
| Electrical Appliances | 6 |
| Home Electrical Appliances | 4 |
| Fabric / Textile / Garment / Home Textile | 4 |
| Chemicals | 4 |
| Bodycare / Cosmetics | 2 |
| Toys and Childcare Products | 2 |
| Sporting Goods / Equipment | 2 |
| Furniture | 2 |
| Other Categories* | 4 |
*Other Categories include Car Accessories, Computer / Audio / Video / Other Electronics & Accessories, Outdoor Living Items and Protective Equipment with a frequency of less than 2.
For a complete list click here
Europe News
The European Union has issued the draft Toy Safety Regulation which repeals 2009/48/EC, the Toy Safety Directive, and introduces stricter safety requirements for toys to be marketed in Europe.
In February 2025, the European Union (EU) issued communication C/2025/1032 outlining its draft Toy Safety Regulation (TSR). This document represents the European Parliament's position, adopted during the first reading in March 2024, aimed at adopting the draft TSR and repealing Directive 2009/48/EC (Toy Safety Directive (TSD)., current consolidated version as of December 2022).
The draft TSR introduces significant revisions to the TSD, including but not limited to:
1. Scope and Exclusions
The regulation applies to all products designed for or intended for play by children under 14 years of age, including products with additional functions beyond play.
Exclusions from Toy Definition:
Scooters designed for children with a body mass exceeding 20 kg.
Books intended for children older than 36 months, provided they are made entirely of paper or cardboard without additional materials.
2. Enhanced Chemical Safety Requirements
Chemical Safety Assessment Enhancements: Manufacturers must consider both individual chemical exposure and combined exposure hazards in toys.
Expansion of Chemical Bans: The general ban on substances classified as carcinogenic, mutagenic, or toxic for reproduction (CMR substances) under Regulation (EC) No. 1272/2008, the regulation that sets requirements for classifying, labeling, and packaging chemical substances and mixtures, is extended to include:
Endocrine disruptors (ED) categories 1 or 2 for human health and the environment.
Specific target organ toxicity (STOT) category 1 (single or repeated exposure).
Respiratory sensitizers category 1.
Skin sensitizers category 1.
Persistent, bioaccumulative, and toxic (PBT); very persistent, very bioaccumulative (vPvB); persistent, mobile, and toxic (PMT); and very persistent, very mobile (vPvM) substances.
Prohibition of Specific Chemicals:
Per- and polyfluoroalkyl substances (PFAS) and bisphenols are banned in toys.
Fragrances are prohibited in toys intended for children under 36 months or those meant to be placed in the mouth.
Chemical Substance Requirement Updates:
Migration limits for 15 elements are introduced. Chromium (VI), cadmium, mercury, and lead are prohibited unless their presence is technically unavoidable under good manufacturing practices (GMP), with each substance not exceeding the limit of detection (LOD).
Nitrosamines and nitrosatable substances in toys are restricted:
≤0.01 mg/kg for nitrosamines.
≤0.1 mg/kg for nitrosatable substances.
Restrictions are imposed on 11 chemicals across all toys. These include TCEP, TCPP, TDCP, formamide, BIT, CMI/MI mixtures, CMI, MI, phenol, formaldehyde, and aniline. Formaldehyde emission limits for wood materials are amended from 0.1 ml/m³ to 0.062 mg/m³.
Allergenic Fragrance Updates:
Allergenic fragrances are permitted only if technically unavoidable under GMP and do not exceed a concentration of 10 mg/kg (reduced from the current limit of 100 mg/kg).
Fragrances exceeding this limit must be listed on the toy's label or accompanying documentation and included in the Digital Product Passport (DPP).
3. Physical and Mechanical Safety Enhancements
Establishes maximum values for both impulse noise and continuous noise emitted by sound producing toys based on scientific studies and recommendation from medical experts.
Special attention is given to toys associated with food, which present unique choking hazards distinct from those posed by toys alone.
Warnings
Safety Pictogram Requirements: Toys unsuitable for children under 36 months must display a pictogram with a minimum diameter of 10 mm and shall contain a red circle with a white background and with the text and face in black color.

All warnings shall be preceded by the word “Warning” or by a generic pictogram which shall be displayed in a prominent way.

5. Digital Product Passport
The DPP will replace the EU declaration of conformity (DoC). It will include product-specific data such as the DoC and additional information accessible electronically via a data carrier.
The Commission is required to adopt delegated acts within 12 months after the date of entry into force (EIF) of the TSR to define technical requirements for the DPP.
The Commission will issue practical guidelines and tailor-made guidance to assist small and medium-sized enterprises (SMEs) in establishing a DPP. An automatic translation tool must be provided for official languages in Member States where the toy is marketed. These resources will be available within one year after the EIF of the TSR.
6. Integration with the General Product Safety Regulation (GPSR)
The TSR is designed to operate complementary to General Product Safety Regulation (EU) 2023/988. Online marketplaces must adhere to Article 22 of Regulation (EU) 2023/988 on product safety.
Transitional Provisions:
The TSD will be repealed on the first day of the month following a transitional period of 30 months after the EIF of the TSR.
Toys compliant with the TSD before its repeal may remain on the market until 50 months after the EIF of the TSR.
On 24 February 2025, the European Union published Commission Implementing Decision (EU) 2025/357 of 21 February 2025, confirming their non-approval of 5-chloro-2-methyl-2H-isothiazol-3-one (CIT) as an active substance for use in biocidal products.
The European Chemicals Agency (ECHA) has reviewed an application received for the approval of 5-chloro-2-methyl-2h-isothiazol-3-one, commonly referred to as CIT, as an active substance for use in biocidal products of product-type 6 (preservatives for products during storage). The application was originally received in 2017 and went through an assessment process which required additional information to be submitted and further reviewed. After completion of the assessment process by the ECHA, the European Union published Commission Implementing Decision (EU) 2025/ 357 of 21 February 2025 which states the non-approval of this substance for use in Type 6 biocidal products. This decision was published on 24 February 2025.
Details as below:
1. Definition
5-chloro-2-methyl-2H-isothiazol-3-one (CIT), CAS number: 26530-03-0, belongs to the isothiazolinone derivatives.
2. Common applications
Preservatives and biocides: used in cosmetics, paints, industrial water treatment, etc., to inhibit microbial growth.
Pharmaceutical intermediates: used as intermediates in the synthesis of other drugs or chemical reagents.
Laboratory research: due to its special chemical structure, it is used in organic synthesis or pharmacological research.
3. Event procedure
Background: On 22 August 2017, the ECHA received an application for the approval of CIT as an active substance for use in biocidal products of product-type 6 (preservatives for products during storage).
Initial Evaluation: In June 2020, the Biocidal Products Committee concluded that the information provided in the application was insufficient to determine whether CIT has endocrine-disrupting properties that may cause adverse effects in non-target organisms.
Revised Assessment: On 22 December 2022, the applicant provided additional data. In September 2023, the evaluating competent authority submitted a revised assessment report, proposing that CIT does not have endocrine-disrupting properties.
ECHA's Opinion: The ECHA discussed the revised assessment report and concluded that the data was still insufficient to determine whether CIT has endocrine-disrupting properties.
Commission's Decision: Based on the ECHA's opinion, the Commission considered that the applicant did not provide sufficient information to determine whether CIT has endocrine-disrupting properties within the prescribed period. The Commission decided not to approve CIT for use in biocidal products of product-type 6.
Entry into Force: The Decision enters into force on the twentieth day following its publication in the Official Journal of the European Union(16 March 2025).
The European Chemical Agency has announced a consultation phase for potential inclusion of three chemicals as Substances of Very High Concern. Should these listings be approved, the number of substances recognized on the candidate list will be updated to 250 entries.
On 28 February 2025, the European Chemical Agency (ECHA) launched a 45-days public consultation on three new potential Substances of Very High Concern (SVHC) as shown in the table as below.
If this is approved, the number of SVHCs on the Candidate List will be updated from 247 entries to 250 entries.
A decision will be made whether these substances will be included in the ECHA Candidate List of SVHC after the consultation.
| Substance name | EC number | CAS number | Reason for inclusion | Possible usage |
|---|---|---|---|---|
| 1,1,1,3,5,5,5-heptamethyl-3-[(trimethylsilyl)oxy]trisiloxane | 241-867-7 | 17928-28-8 | vPvB* (Article 57e) | Cosmetics and personal care products Perfumes and fragrances |
| Decamethyltetrasiloxane | 205-491-7 | 141-62-8 | vPvB* (Article 57e) | Cosmetics and personal care products Non-metal-surface treatment products Lubricants, greases, release products Automotive care products Washing and cleaning products (including solvent based products) |
| Tetra(sodium/potassium) 7-[(E)-{2-acetamido-4-[(E)-(4-{[4-chloro-6-({2-[(4-fluoro-6-{[4-(vinylsulfonyl)phenyl]amino}-1,3,5-triazine-2-yl)amino]propyl}amino)-1,3,5-triazine-2-yl]amino}-5-sulfonato-1-naphthyl)diazenyl]-5-methoxyphenyl}diazenyl]-1,3,6-naphthalenetrisulfonate; Reactive Brown 51 | 466-490-7 | / | Toxic for reproduction (Article 57c) | Textile treatment products and dyes |
*Remark:
VPvB = very Persistent and very Bioaccumulative
Companies should assess the impact of the addition of these chemicals and be prepared with potential alternative options.
As the latest amendment of European food contact plastic related regulations, new Regulation (EU) 2025/351 clarifies a number of ambiguities and requires more from the supply chain.
On 21 February 2025, the European Commission released Regulation (EU) 2025/351. This Regulation applies to plastic materials for articles intended to come into contact with food and acts as an amendment to other regulations by clarifying a number of ambiguities. It also focuses additional responsibilities on the supply chain.
Details as below:
1. Relationship with related regulations:
Amends Regulation (EU) No 10/2011 on food contact plastic materials and articles
Amends Regulation (EU) 2022/1616 on recycled plastic materials and articles intended to come into contact with foods
Amends Regulation (EC) No 2023/2006 on good manufacturing practices for food contact materials and articles
Repeals Regulation (EC) No 282/2008 on food contact recycled plastic materials and articles
2. Main updates:
A new article of high degree of purity is added to (EU) 10/2011
To ensure better alignment of Regulation (EU) No 10/2011 with Regulation (EC) No 1907/2006 (the European Union's (EU) Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation), specific rules are laid down regarding the purity of substances of natural origin, so-called Unknown or Variable Composition, Complex Reaction Products, or Biological Materials (UVCB) substances.
Regulation (EU) No 10/2011 does not impose restrictions on the source of substances that can be used in the manufacture of plastic materials and articles, therefore, such substances may be manufactured from waste. It is required that substances produced from waste should also be of a high level of purity.
Additional requirements for supporting documents in supply chain
a.) Documents: For substances used in the manufacture of plastic materials and articles, documentation on the composition shall be provided on request, together with any documentation regarding purity.
b.) Sampling: Manufacturers of plastic materials and articles, and of products from intermediate stages, shall ensure that competent authorities can take samples during the carrying out of official controls to verify their degree of purity and their composition, including that of the substances and materials used for their manufacture.
A new section of reprocessing of plastics is added to Regulation (EC) No 2023/2006
Plastic offcuts, scraps, and similar by-products of plastic manufacturing processes intended to be reprocessed into food contact plastics, shall be collected separately from waste.
At any stage of the reprocessing of plastic, operators shall ensure that the quality assurance system prevents it from being mixed with plastic of a different composition, other materials, or with waste.
This regulation will become effective on 16 March 2025. It is imperative for relevant businesses to know about the updated regulatory requirements to ensure their production and processes will be compliant.
This draft bill represents the standpoint and actions of PFAS restriction in France, aiming to align with the RO1/RO2 bans by ECHA PFAS proposal.
In Europe, initiatives are moving in the direction of a broad restriction of PFAS. The draft European ban on PFAS published by ECHA in 2023 and prepared by Germany, the Netherlands, Norway, Sweden and Denmark since 2021, France is not at the origin of this restriction project but has recently given its support.
However, this provision in France will ensure the application of a broad ban on national territory, regardless of the uncertainties of the European decision-making process.
Main contents of this draft bill:
1. Prohibiting the manufacture, import, export and placing on the market of products containing PFAS.
Four usages are targeted for a ban from 1 July 2025:
Food contact products
Cosmetics
Wax
Textile products (PPE excepted)
Any product containing PFAS shall be prohibited as from 1 July 2027
2. Proposing to integrate PFAS into the control of water intended for human consumption. Drinking water, a major source of exposure to PFAS, is currently not subject to PFAS control.
Proposing to integrate PFAS into the control of water intended for human consumption. Drinking water, a major source of exposure to PFAS, is currently not subject to PFAS control.
3. France intended to ban PFAS in a systemic manner, not just targeted restrictions, such as those for PFOS and PFOA.
This draft bill will take effect the day after it’s officially published.
In Europe, when hazards are identified in non-food consumer products, the products will be recalled and published in the Safety Gate system, which is updated weekly. The European recalls from 01 February 2025 to 28 February 2025 are summarized below:
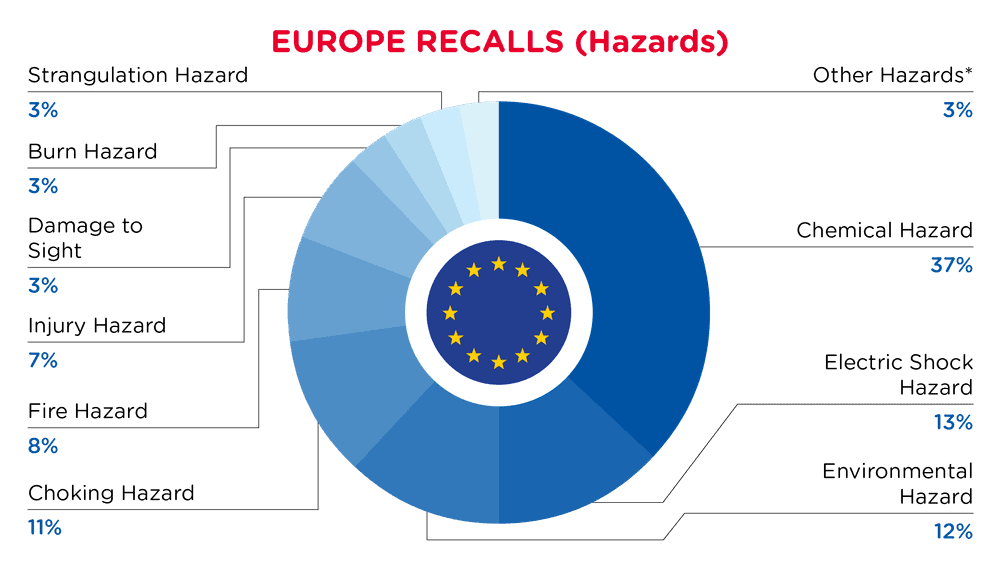
| Hazards | Frequency |
| Chemical Hazard | 124 |
| Electric Shock Hazard | 43 |
| Environmental Hazard | 41 |
| Choking Hazard | 37 |
| Fire Hazard | 26 |
| Injury Hazard | 25 |
| Damage to Sight | 10 |
| Burn Hazard | 9 |
| Strangulation Hazard | 9 |
| Other Hazards* | 11 |
*Other Hazards include Damage to Hearing, Microbiological Hazard, Cut Hazard, Suffocation Hazard and Safety Risk Hazard with a frequency of less than 5.

| Product Categories | Frequency |
| Toys and Childcare Products | 76 |
| Bodycare / Cosmetics | 64 |
| Electrical Appliances | 62 |
| Chemicals | 17 |
| Fabric / Textile / Garment / Home Textile | 11 |
| Jewelry | 9 |
| Sporting Goods / Equipment | 6 |
| Protective Equipment | 6 |
| Household Items | 5 |
| Other Categories* | 26 |
*Other Categories include Tools and Hardware, Outdoor Living Items, Home Electrical Appliances, Food Contact Material, Machinery, Computer / Audio / Video / Other Electronics & Accessories, Footwear, Accessories, Travel Items, Furniture and Car Accessories with a frequency of less than 5.

| Notifying Country | Frequency |
| Sweden | 48 |
| Czechia | 31 |
| France | 26 |
| Italy | 26 |
| Germany | 23 |
| United Kingdom in respect of Northern Ireland | 21 |
| Hungary | 21 |
| Spain | 13 |
| Finland | 11 |
| Croatia | 10 |
| Belgium | 8 |
| Poland | 7 |
| Ireland | 6 |
| Lithuania | 6 |
| Other Countries* | 25 |
*Other Countries include Estonia, Slovakia, Malta, Bulgaria, Slovenia, Austria, The Netherlands, Cyprus, Romania, Latvia and Denmark with a frequency of less than 6.
For a complete list click here
China News
In China, when hazards are identified in consumer products, they will be recalled and published in the SAMR Defective Product Administrative Centre, which is updated daily. The China recalls from 01 February 2025 to 28 February 2025 are summarized below:
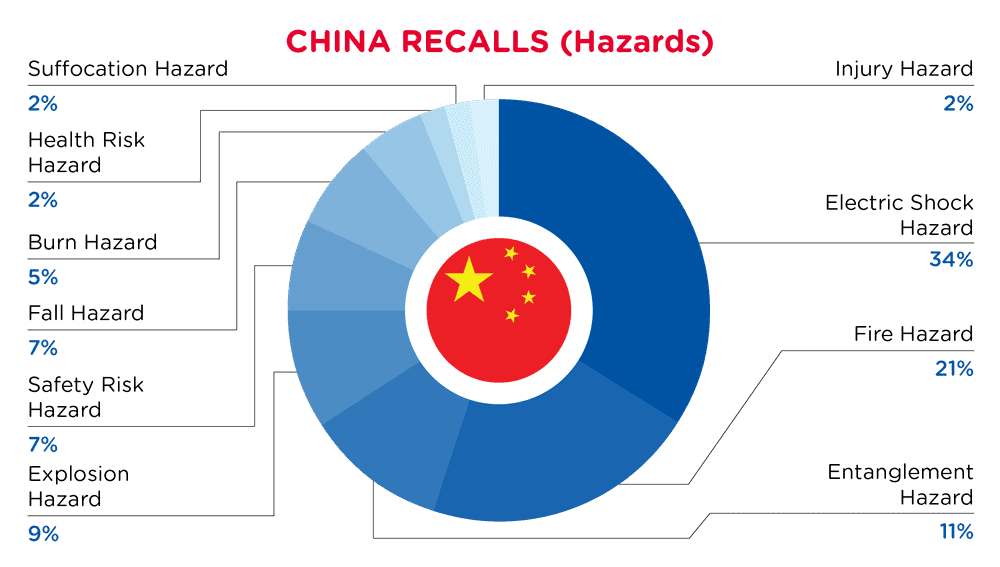
| Hazards | Frequency |
| Electric Shock Hazard | 15 |
| Fire Hazard | 9 |
| Entanglement Hazard | 5 |
| Explosion Hazard | 4 |
| Safety Risk Hazard | 3 |
| Fall Hazard | 3 |
| Burn Hazard | 2 |
| Health Risk Hazard | 1 |
| Suffocation Hazard | 1 |
| Injury Hazard | 1 |

| Product Categories | Frequency |
| Home Electrical Appliances | 12 |
| Fabric / Textile / Garment / Home Textile | 5 |
| Sporting Goods / Equipment | 5 |
| Electrical Appliances | 3 |
| Toys and Childcare Products | 2 |
| Stationery | 1 |
| Footwear | 1 |
| Furniture | 1 |
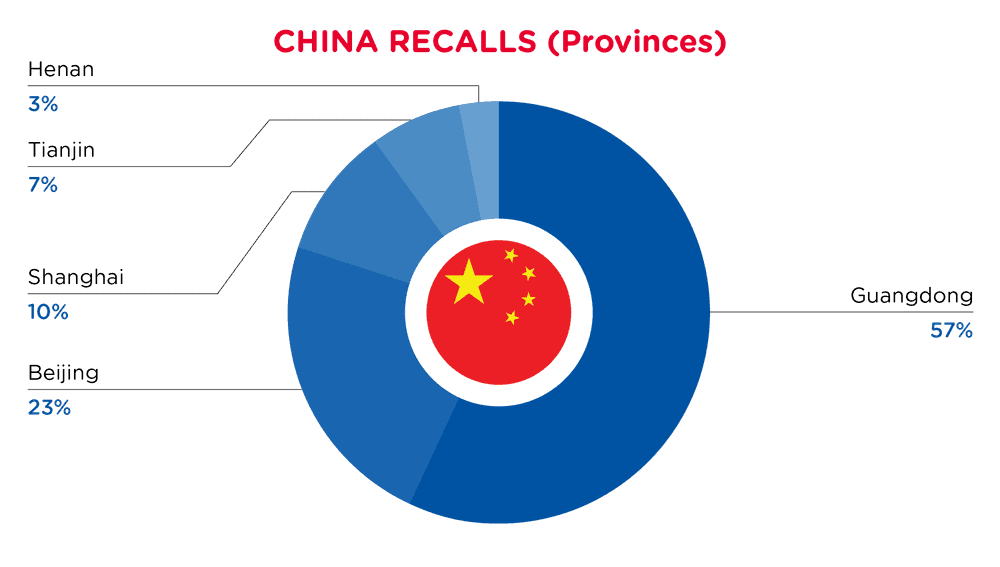
| Provinces | Frequency |
| Guangdong | 17 |
| Beijing | 7 |
| Shanghai | 3 |
| Tianjin | 2 |
| Henan | 1 |
For a complete list click here
Australia/New Zealand News
In Australia, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Australian Competition & Consumer Commission website, which is updated daily. The Australia recalls from 01 February 2025 to 28 February 2025 are summarized below:
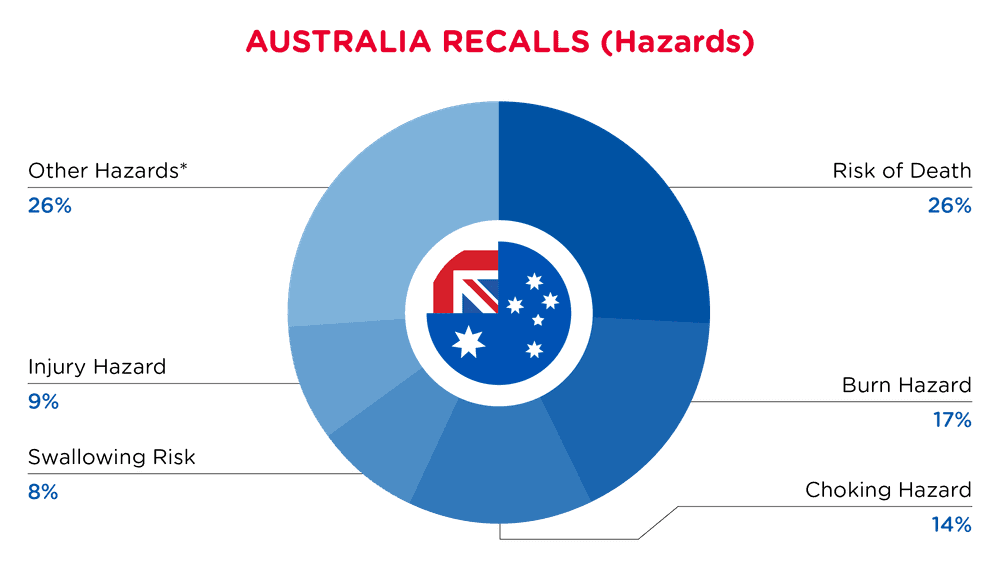
| Hazards | Frequency |
| Risk of Death | 9 |
| Burn Hazard | 6 |
| Choking Hazard | 5 |
| Swallowing Risk | 3 |
| Injury Hazard | 3 |
| Other Hazards* | 9 |
*Other Hazards include Crushing Hazard, Cut Hazard, Eye Irritation Risk, Drowning Hazard, Amputation Hazard, Strangulation Hazard, Fire Hazard, Skin Irritation Risk and Fall Hazard with a frequency of less than 2.

| Product Categories | Frequency |
| Toys and Childcare Products | 4 |
| Accessories | 2 |
| Furniture | 2 |
| Pet Items | 1 |
| Computer / Audio / Video / Other Electronics & Accessories | 1 |
| Outdoor Living Items | 1 |
| Fabric / Textile / Garment / Home Textile | 1 |
| Household Items | 1 |
| Sporting Goods / Equipment | 1 |
| Bodycare / Cosmetics | 1 |
For a complete list click here
Subscribe to our Regulatory Updates
Unsubscribe at any time. Read our privacy policy.