SFDA Announcement
From October 3rd 2021, all importers must request a Certificate of Compliance for their cosmetics shipment via FASEH: https://faseh.sfda.gov.sa/, and once the request has been received, reviewed and assessed by QIMA, we will print and upload the certificate as soon as compliance is verified.
Saudi Food and Drug Authority (SFDA) is primarily responsible for regulating, overseeing, and monitoring food, cosmetics, drugs, and medical devices, as well as establishing mandatory requirements for regulated products. Food, drugs, medical devices, cosmetics, pesticides, and feed are all regulated by SFDA.
It is mandatory for cosmetic products to comply with the SFDA GSO 1943 regulation on Cosmetics and Personal Care products. A list of prohibited substances as well as approved preservatives, UV filters, and colorants is also included in the annexes. Additionally, importers need to ensure that products adhere to requirements published in Circulars issued by the SFDA.
The SFDA defines cosmetic products as those that are intended to be placed into contact with the external parts of the human body such as the skin, hair system, nails, lips, including teeth and mucous membranes of the mouth. Products that are intended for internal uses are not included in scope.
QIMA, a leading provider of quality control and supply chain compliance solutions, announced in April 2022 that it has been approved as a Notified Body by the Saudi Food and Drug Authority (SFDA) to certify cosmetic products being imported or sold in the Kingdom of Saudi Arabia. QIMA is equipped to provide all additional necessary services to help exporters secure product entry into Saudi Arabia including:
QIMA will test the product samples.
Risk Assessment for importers or manufacturers unsure of the risk assessment requirement.
Label review to help brands avoid delays during the conformity assessment process.
Need more information?
By contacting QIMA you agree to our privacy policy and terms and conditions.
Certification Process
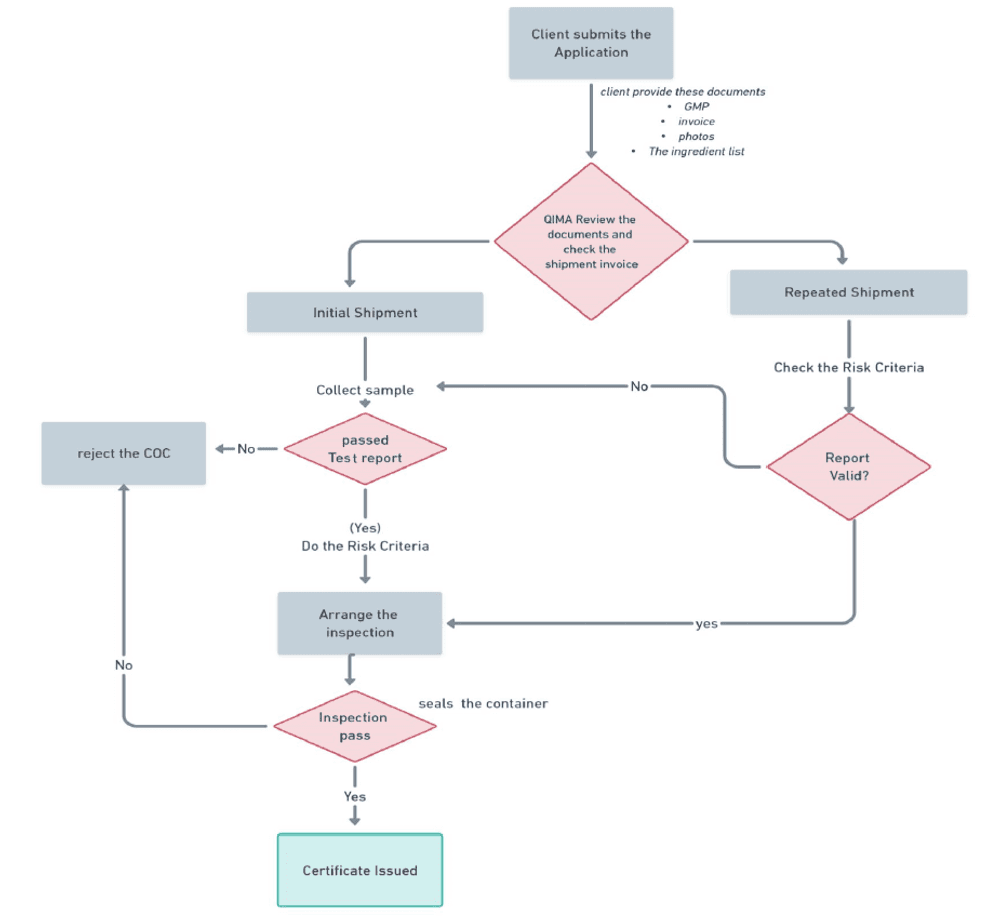
Testing
SFDA requires cosmetic products to be compliant with SFDA.CO/GSO 1943:2016.
SFDA.CO/GSO 1943:2016 describes the chemical safety, labelling, product claims, and packaging requirements for cosmetic products.
Requirements set forth in circular published by SFDA
Registration
The cosmetic products notification system (Alghad) is where importers register products that they intend to import.
All required information and documentation shall be uploaded to Alghad. Licenses and notification certificates are issued through the system.
Registration on Alghad consists of 3 parts:
Creating an importer account
Register for manufacturer (there can be more than one manufacturer linked to each account)
Product notification
Note: If products are to be stored in a warehouse before being distributed in the market, the warehouse shall be registered as well on Alghad.
CoC
After obtaining an Alghad notification certificate, the importer can request a CoC from an approved certification body. The CoC is needed for clearance of the product from Saudi Customs.
